Understanding Charging (充電)and Discharging (放電)
Electrochemistry - using electricity to drive chemical reactions and then through chemical reactions to make electricity !!!
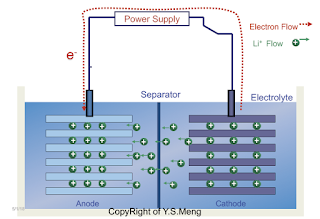
One of the most difficult concepts I teach for electrochemistry class, is the concept of VOLTAGE, a.k.a. electric potential difference or some old text books call it "an electromotive force". If one considers a generic picture of a battery, consisting a negative electrode, a positive electrode and the electrolyte. Electrons flow (outside the battery) from the cathode (lithium transition metal oxide) to anode (graphite) during the charging of the battery, lithium ions flow from the cathode to the anode INSIDE the battery through the electrolyte. It may be intuitive for people to think the battery as a blackbox storing full of electrons - but such understanding is so conceptually inaccurate.
Charging (when you plug in your phone) - you are using the electricity from to make chemical reactions in the battery. (Voltage will be raised during this process - two very unstable compounds will be made: lithiated graphite (C6Lix) and delithiated lithium transition metal oxides ( Li1-xCoO2)
One of the most difficult concepts I teach for electrochemistry class, is the concept of VOLTAGE, a.k.a. electric potential difference or some old text books call it "an electromotive force". If one considers a generic picture of a battery, consisting a negative electrode, a positive electrode and the electrolyte. Electrons flow (outside the battery) from the cathode (lithium transition metal oxide) to anode (graphite) during the charging of the battery, lithium ions flow from the cathode to the anode INSIDE the battery through the electrolyte. It may be intuitive for people to think the battery as a blackbox storing full of electrons - but such understanding is so conceptually inaccurate.
Charging (when you plug in your phone) - you are using the electricity from to make chemical reactions in the battery. (Voltage will be raised during this process - two very unstable compounds will be made: lithiated graphite (C6Lix) and delithiated lithium transition metal oxides ( Li1-xCoO2)
Electrons are only generated because ions move from high chemical potential to low chemical potential (Voltage will be lowered during this process) - these electrons can light up your electric appliances for a certain period of time. Therefore, I say in my class that ion mobility and electron mobility must be considered and very often ion mobility is the weakest link in battery performances.

Comments
Post a Comment